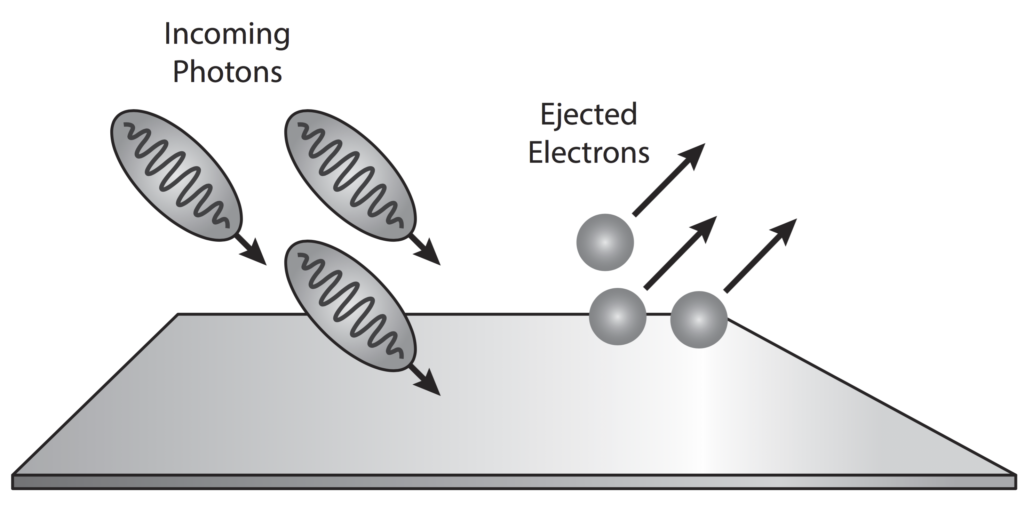
Light Quantum Behavior . there is one lucky break, however—electrons behave just like light. Describe the relationship between wavelength, frequency, and speed of light. The quantum behavior of atomic objects (electrons, protons, neutrons,. Discuss the particle model of light and the definition of photon. explain the evidence for maxwell’s electromagnetic model of light. the quantum theory of light elucidates light's duality as both waves and particles with quantized energy levels. einstein's introduction of photons was the first step toward a true quantum theory of light—just as the bohr model of the atom. einstein, planck, and compton showed the explanatory power of giving light a discrete or quantum interpretation. Originating from max planck's energy. Explain how and why the amount of light we see from an object depends upon its distance.
from scottbembenek.com
Describe the relationship between wavelength, frequency, and speed of light. the quantum theory of light elucidates light's duality as both waves and particles with quantized energy levels. einstein, planck, and compton showed the explanatory power of giving light a discrete or quantum interpretation. Discuss the particle model of light and the definition of photon. there is one lucky break, however—electrons behave just like light. The quantum behavior of atomic objects (electrons, protons, neutrons,. Originating from max planck's energy. Explain how and why the amount of light we see from an object depends upon its distance. einstein's introduction of photons was the first step toward a true quantum theory of light—just as the bohr model of the atom. explain the evidence for maxwell’s electromagnetic model of light.
The Photoelectric Effect Scott D. Bembenek
Light Quantum Behavior Originating from max planck's energy. einstein, planck, and compton showed the explanatory power of giving light a discrete or quantum interpretation. the quantum theory of light elucidates light's duality as both waves and particles with quantized energy levels. The quantum behavior of atomic objects (electrons, protons, neutrons,. Describe the relationship between wavelength, frequency, and speed of light. Explain how and why the amount of light we see from an object depends upon its distance. explain the evidence for maxwell’s electromagnetic model of light. Discuss the particle model of light and the definition of photon. there is one lucky break, however—electrons behave just like light. einstein's introduction of photons was the first step toward a true quantum theory of light—just as the bohr model of the atom. Originating from max planck's energy.
From www.wired.com
The Weird Quantum Behavior of Light, Captured in a Lab WIRED Light Quantum Behavior Explain how and why the amount of light we see from an object depends upon its distance. Describe the relationship between wavelength, frequency, and speed of light. The quantum behavior of atomic objects (electrons, protons, neutrons,. there is one lucky break, however—electrons behave just like light. einstein, planck, and compton showed the explanatory power of giving light a. Light Quantum Behavior.
From newatlas.com
"Solid" light reveals new insights about quantum mechanics Light Quantum Behavior The quantum behavior of atomic objects (electrons, protons, neutrons,. Describe the relationship between wavelength, frequency, and speed of light. there is one lucky break, however—electrons behave just like light. einstein's introduction of photons was the first step toward a true quantum theory of light—just as the bohr model of the atom. Discuss the particle model of light and. Light Quantum Behavior.
From www.slideserve.com
PPT Light Waves and Polarization PowerPoint Presentation ID6103736 Light Quantum Behavior Originating from max planck's energy. einstein's introduction of photons was the first step toward a true quantum theory of light—just as the bohr model of the atom. einstein, planck, and compton showed the explanatory power of giving light a discrete or quantum interpretation. The quantum behavior of atomic objects (electrons, protons, neutrons,. the quantum theory of light. Light Quantum Behavior.
From interestingengineering.com
Quantum Tunneling Is Absolutely Bonkers, Here Is What You Need to Know Light Quantum Behavior Originating from max planck's energy. einstein, planck, and compton showed the explanatory power of giving light a discrete or quantum interpretation. the quantum theory of light elucidates light's duality as both waves and particles with quantized energy levels. Describe the relationship between wavelength, frequency, and speed of light. Discuss the particle model of light and the definition of. Light Quantum Behavior.
From www.livescience.com
Quantum Mystery of Light Revealed by New Experiment Live Science Light Quantum Behavior einstein's introduction of photons was the first step toward a true quantum theory of light—just as the bohr model of the atom. Describe the relationship between wavelength, frequency, and speed of light. explain the evidence for maxwell’s electromagnetic model of light. Explain how and why the amount of light we see from an object depends upon its distance.. Light Quantum Behavior.
From studylib.net
Polarized Light and Quantum Mechanics Light Quantum Behavior Explain how and why the amount of light we see from an object depends upon its distance. einstein's introduction of photons was the first step toward a true quantum theory of light—just as the bohr model of the atom. Describe the relationship between wavelength, frequency, and speed of light. Originating from max planck's energy. einstein, planck, and compton. Light Quantum Behavior.
From blogs.ntu.edu.sg
Probing the Ultimate Limits of Quantum Detection Science NTU Light Quantum Behavior there is one lucky break, however—electrons behave just like light. Discuss the particle model of light and the definition of photon. explain the evidence for maxwell’s electromagnetic model of light. Describe the relationship between wavelength, frequency, and speed of light. Explain how and why the amount of light we see from an object depends upon its distance. . Light Quantum Behavior.
From courses.lumenlearning.com
Development of Quantum Theory Chemistry for Majors Light Quantum Behavior Discuss the particle model of light and the definition of photon. Originating from max planck's energy. einstein's introduction of photons was the first step toward a true quantum theory of light—just as the bohr model of the atom. einstein, planck, and compton showed the explanatory power of giving light a discrete or quantum interpretation. Describe the relationship between. Light Quantum Behavior.
From thecreatorsproject.vice.com
Quantum Theory A Playable Physical Object Creators Light Quantum Behavior Explain how and why the amount of light we see from an object depends upon its distance. einstein's introduction of photons was the first step toward a true quantum theory of light—just as the bohr model of the atom. there is one lucky break, however—electrons behave just like light. The quantum behavior of atomic objects (electrons, protons, neutrons,.. Light Quantum Behavior.
From www.blog.sindibad.tn
Quantum Field Theory visualized Magic of Science Light Quantum Behavior einstein, planck, and compton showed the explanatory power of giving light a discrete or quantum interpretation. there is one lucky break, however—electrons behave just like light. Discuss the particle model of light and the definition of photon. Explain how and why the amount of light we see from an object depends upon its distance. einstein's introduction of. Light Quantum Behavior.
From news.mit.edu
Generating highquality single photons for quantum computing MIT News Light Quantum Behavior einstein's introduction of photons was the first step toward a true quantum theory of light—just as the bohr model of the atom. explain the evidence for maxwell’s electromagnetic model of light. the quantum theory of light elucidates light's duality as both waves and particles with quantized energy levels. einstein, planck, and compton showed the explanatory power. Light Quantum Behavior.
From www.slideserve.com
PPT Matter and Particles of Light Quantum Theory PowerPoint Light Quantum Behavior Originating from max planck's energy. einstein, planck, and compton showed the explanatory power of giving light a discrete or quantum interpretation. The quantum behavior of atomic objects (electrons, protons, neutrons,. there is one lucky break, however—electrons behave just like light. Discuss the particle model of light and the definition of photon. Describe the relationship between wavelength, frequency, and. Light Quantum Behavior.
From www.quantumbiology.gr
Probing Vision with Quantum Light Quantum Physics & Quantum Biology Light Quantum Behavior The quantum behavior of atomic objects (electrons, protons, neutrons,. einstein's introduction of photons was the first step toward a true quantum theory of light—just as the bohr model of the atom. Discuss the particle model of light and the definition of photon. einstein, planck, and compton showed the explanatory power of giving light a discrete or quantum interpretation.. Light Quantum Behavior.
From www.studypool.com
SOLUTION Quantum physics duality of light Studypool Light Quantum Behavior Describe the relationship between wavelength, frequency, and speed of light. Originating from max planck's energy. explain the evidence for maxwell’s electromagnetic model of light. the quantum theory of light elucidates light's duality as both waves and particles with quantized energy levels. The quantum behavior of atomic objects (electrons, protons, neutrons,. Discuss the particle model of light and the. Light Quantum Behavior.
From www.slideserve.com
PPT Is Matter Made of Light? Superluminal Quantum Models of the Light Quantum Behavior Originating from max planck's energy. the quantum theory of light elucidates light's duality as both waves and particles with quantized energy levels. there is one lucky break, however—electrons behave just like light. einstein, planck, and compton showed the explanatory power of giving light a discrete or quantum interpretation. Explain how and why the amount of light we. Light Quantum Behavior.
From www.studypool.com
SOLUTION Einstein s quantum theory of light Studypool Light Quantum Behavior Explain how and why the amount of light we see from an object depends upon its distance. einstein's introduction of photons was the first step toward a true quantum theory of light—just as the bohr model of the atom. there is one lucky break, however—electrons behave just like light. explain the evidence for maxwell’s electromagnetic model of. Light Quantum Behavior.
From physicsinmyview.com
Wave Particle Duality Physical Reality of Quantum Physics Physics Light Quantum Behavior Describe the relationship between wavelength, frequency, and speed of light. Originating from max planck's energy. einstein's introduction of photons was the first step toward a true quantum theory of light—just as the bohr model of the atom. Discuss the particle model of light and the definition of photon. The quantum behavior of atomic objects (electrons, protons, neutrons,. explain. Light Quantum Behavior.
From scottbembenek.com
The Photoelectric Effect Scott D. Bembenek Light Quantum Behavior Discuss the particle model of light and the definition of photon. Describe the relationship between wavelength, frequency, and speed of light. Explain how and why the amount of light we see from an object depends upon its distance. there is one lucky break, however—electrons behave just like light. einstein's introduction of photons was the first step toward a. Light Quantum Behavior.